Viruses change continuously. These changes are based on random mutations. For the SARS-CoV-2 coronavirus, mutation-induced changes enabled it to infect humans. Meanwhile, new mutations in SARS-CoV-2 are again jeopardizing initial achievements against the virus, such as vaccines that have been developed. It's a race between virus and human. An excellent reason for the German Research Platform for Zoonoses to take a closer look at the topic of mutations in a conversation with virus researcher Dr. Imke Steffen.
Dr. Imke Steffen is a member of the internal advisory board of the Zoonoses Platform and heads a junior research group at the TiHo Hannover, which, among other things, deals with emerging RNA viruses and their interaction with human and animal hosts. Among other things, the Zoonoses Platform spoke with her about the questions of what mutations are, how they arise and what role the environment plays.
„a mutation is a change in genetic information“
ZOOP: Ms. Steffen, first of all, thank you for taking the time to answer our questions. Let's just jump into the topic of mutations with the question, "What is a mutation?"
Steffen: Mutations are changes in the genetic sequence. Here, we distinguish between different types of mutations. On the one hand, there are point mutations, in which individual bases of the sequence are exchanged. Then there are other forms in which larger areas are changed. Generally speaking, however, a mutation is a change in genetic information.
ZOOP: How do mutations occur? How can they come about?
Steffen: It generally happens when genetic information is copied by viruses, or even by higher organisms, in the course of reproduction. Errors can occur during these copying processes because the enzymes involved in the process do not work without errors. As a result, changes can be inserted into the genome so that the copied sequence differs from the original sequence.
ZOOP: Are these always just individual small changes or can they also be larger?
Steffen: That depends on what type of mutation is present. In point mutations, individual bases are inserted incorrectly and lead to changes that can solidify in the genome. In so-called recombinations, entire regions of the genome are exchanged. This can occur because the enzyme responsible for the copying process changes the template for the copy within the process. This can happen when there are multiple copies of a virus within a cell and the enzyme combines two of these copies as a template. In such a recombination, entire regions of the genome can be altered.
ZOOP: To find mutations, there is the possibility of sequencing, i.e. looking at the complete base sequence of a genome and examining it for changes. Another option are PCRs (polymerase chain reactions), which can be used to amplify parts of a genome. How exactly does that work?
Steffen: Great progress has been made in sequencing in recent years, so that it is now possible to identify different genome variants from different sequences. However, sequencing still involves a relatively large amount of work. In diagnostics, therefore, PCR is more commonly used, which allows one to specifically amplify and enrich fragments of a genome, so that one can then sequence specific sections and detect certain mutations in this way. It is also possible to design the PCR itself in such a way that certain variants can be detected and others not. This is called a type-specific PCR. For this purpose, the so-called primers (short segments that bind to certain sequence segments) are designed in such a way that they can only bind to the sequences of certain variants. In this way, it is possible to distinguish between different variants. A prerequisite for this is, of course, that one knows the mutations one is looking for. The method is not suitable for finding unknown new mutations, but one must know the differences on a sequence basis.
„the genom of RNA viruses changes about 1 million times faster than the human“
ZOOP: The mutation rate in RNA viruses, i.e. the rate at which changes occur in the genome, differs from the mutation rate in human cells. What is the reason for this?
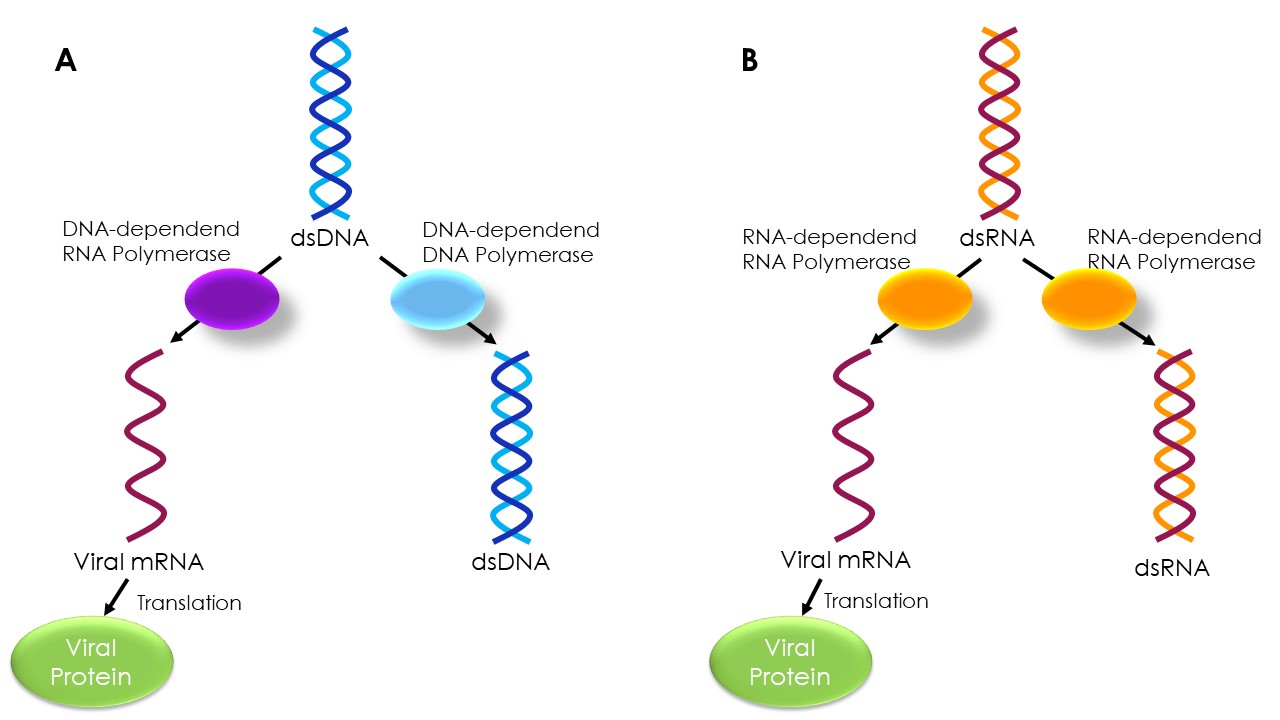
Steffen: In fact, the genome of RNA viruses is thought to change about 1 million times faster than the human genome. One reason for this is that RNA is not as easy to copy. The human genome consists of DNA, which is much more stable than RNA. In addition, the enzymes that amplify DNA, known as DNA polymerases, work more precisely than RNA polymerases. Viruses usually code for their own RNA-dependent RNA polymerase, since this is not present in host cells. In human cells, RNA is produced based on DNA. The process of creating RNA based on an RNA template is a distinctive feature of RNA viruses. However, unlike DNA polymerases, these RNA polymerases do not have their own proof-reading function, which can correct any errors that arise in the newly copied sequence if necessary. As a result, more errors occur during the copying process from RNA to RNA than during the copying of DNA molecules.
Replication and translation of double-stranded (ds) RNA and DNA viruses A) Viruses whose genome is present as dsDNA require a DNA-dependent DNA polymerase to copy the genetic information. Translation of the genetic information into a protein occurs via a DNA-dependent RNA polymerase to a messenger RNA (mRNA). B) In RNA viruses, the genome is present as RNA. Therefore, other enzymes are required for replication and translation. Alternatively, the genetic information in viruses may be present as a single-stranded DNA or RNA molecule. The error rate in the replication process differs between the different genome types.
ZOOP: Does it affect the mutation rate whether the DNA or RNA is single- or double-stranded?
Steffen: Both DNA and RNA viruses can have double-stranded or single-stranded genomes. In fact, it seems that double-stranded genomes offer some protection against mutations compared to the single-stranded ones. So genome structure also plays a role.
"Coronaviruses tend to have a lower mutation rate compared to other RNA viruses"
ZOOP: How does the mutation rate of coronaviruses compare with other RNA viruses?
Steffen: Coronaviruses are actually special within RNA viruses because they have the longest genome compared to other RNA viruses. Normally, the mutation rate limits the size of the genome, because as the genome size increases, the number of errors per copy can naturally increase. Coronaviruses can only afford such a large genome because they encode an additional enzyme that performs the corrective function discussed earlier. For this reason, coronaviruses tend to have a lower mutation rate compared with other RNA viruses.
"It is important to realize that mutations happen randomly, initially as a single case"
ZOOP: Considering that some mutations can also have negative effects on viral survivability, can you actually determine the mutation rate in the laboratory? Can you also detect lethal mutations?
Steffen: It's actually not that simple. In the laboratory, there are different methods to determine mutation rates. These range from determining the pure activity of the isolated polymerase and its error rate to looking at entire genomes. However, most methods are biased towards functional mutations, which then also occur with a certain frequency. One must realize that mutations happen randomly, initially as a single case. Only certain mutations can manifest themselves in a virus population, which always consists of different variants. Depending on the method, there are detection limits for the detection of single mutations. Lethal mutations are often underrepresented in such methods. However, one can mathematically correct for this when calculating the mutation rate. However, this is not my area of expertise. But determining mutation rates under laboratory conditions is not easy in any case, because some environmental factors are omitted here.
ZOOP: Besides the mutation rate, what other factors determine the genetic diversity of a population?
Steffen: There are a number of factors to be mentioned here. For example, the size of the population plays a role, influencing both the number of variants within a population and the probability that a mutation will become established. The host also plays a major role. Through its immune response, it helps determine which viruses are well adapted. The effects of co-evolution should not be neglected here either. In addition, host switching can play a role. Arboviruses (vector-borne viruses), for example, switch between mammals and arthropods, which are very different from each other. This has an impact on diversity. In addition, selection pressure in general plays a role. Host-to-host transmission, for example, is always a bottleneck for a virus population, which is usually only mastered by a few variants, which in turn can then establish a new virus population.
ZOOP: Not every mutation changes the properties of a virus. What conditions must be met for a mutation to result in altered properties?
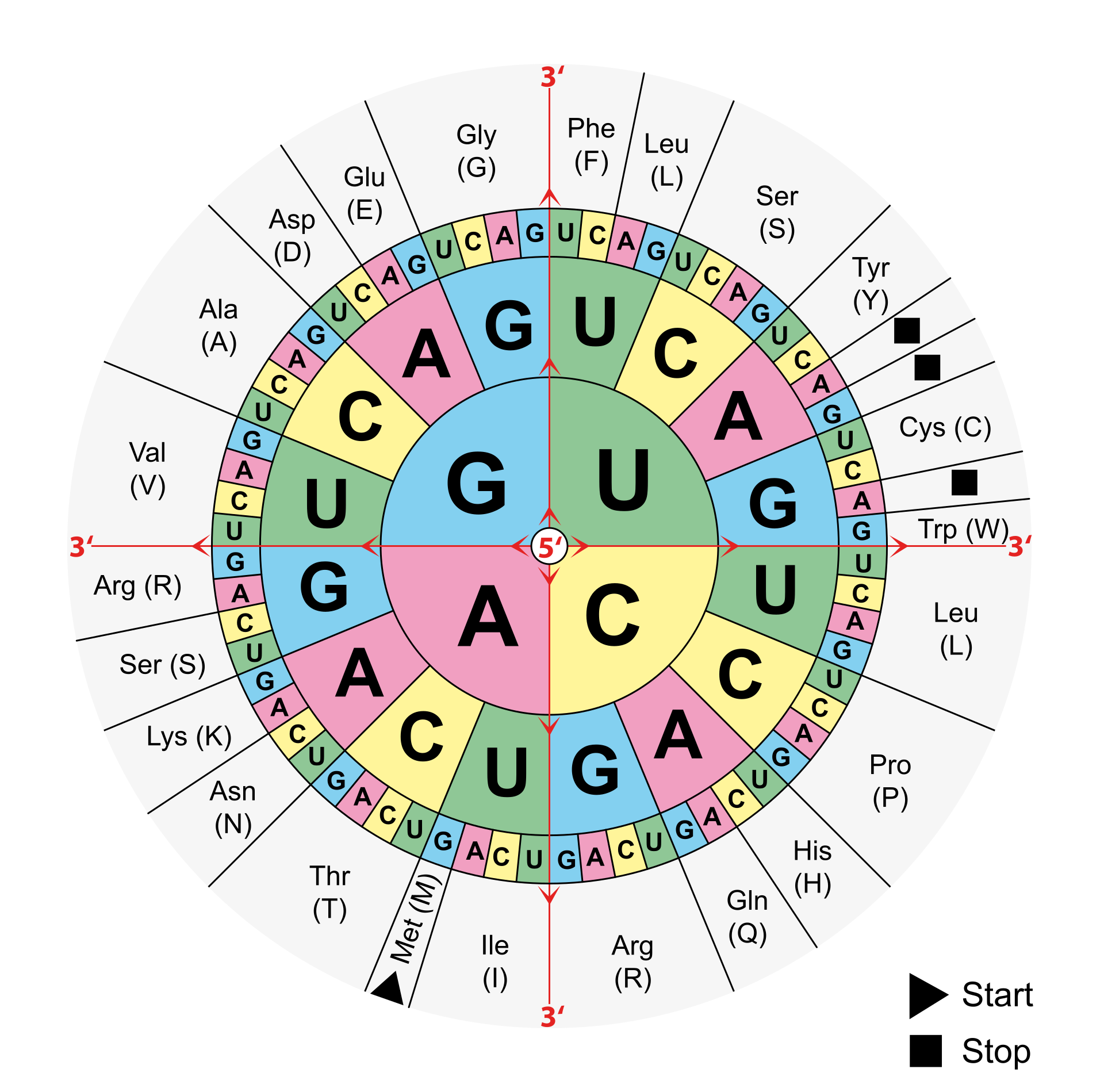
Steffen: First, it is important to know that three nucleotides code for one amino acid. However, these three nucleotides, known as codons, are redundant, i.e. there are several codons that code for the same amino acid. Not every change at the level of DNA or RNA therefore manifests itself in a changed amino acid in the protein that is formed at the end. These are referred to as silent mutations. Other point mutations can lead to the formation of so-called stop codons, which can lead to an abortion of the reading process. If a mutation does lead to an amino acid exchange in the protein, this does not necessarily have an effect on its function. It depends very much on the position of the protein at which the exchange occurs and which amino acids are exchanged for each other.
Code sun for the genetic code. Three nucleotides each encode an amino acid from which a protein is formed at the end. The nucleic bases are read from the inside to the outside, the respective amino acids or the stop signals can be read at the edge. (grafic: Mouagip)
"The viral genome is reduced to the essentials"
ZOOP: In humans, not all genome segments code for proteins. What is the situation with viruses? Are there also sections in their genome that seem to be less relevant?
Steffen: Viruses are more efficient in that respect. Their genome is reduced to the essentials. That is why they need the host cell to reproduce. Often they use enzymes of the cells, which they do not encode in their own genome. Some genomes code for only 4 or 5 proteins, but these are sufficient to carry out the replication process. There are various mechanisms in viruses to encode as much as possible within this limited genome size. So the viral genome is really very compact and that is just because the genome size is limited by the mutation rate. In a large genome, too many detrimental mutations could accumulate. In the lab, you notice this when you try to insert certain sequences, a dye, for example. Many viruses have a tendency to quickly eliminate these sequences from their genome, since they have no direct advantage from doing so.
ZOOP: Looking at the interaction between mutations and the environment, can we draw a blanket conclusion that high mutation rates tend to be helpful in a dynamic environment, while they tend not to be favored under stable environmental conditions?
Steffen: First of all, mutation rates are independent of the environment. The substitution rate (rate at which mutations manifest themselves in a population), on the other hand, is co-determined by environmental influences. In the case of arboviruses, the environmental changes that occur as a result of host switching tend to result in stable populations, since each host switch reduces the diversity of a population again. When a virus is free to replicate without interference from an immune system, random mutations can occur and may become entrenched in a population. Now, when an immune system comes into play, it will be observed that the selection of mutations is shifted in a certain direction. It is an interplay between positive and negative selection, which may well be influenced by the environment. The mutation rate itself remains the same because it is determined by other factors.
"Mutations that lead to higher fitness are principally advantageous"
ZOOP: What determines whether a mutant will prevail in a population? Does the best adaptation win?
Steffen: On the one hand, fitness plays a role here, which describes the speed of reproduction. Mutations that lead to a higher fitness are principally advantageous. In addition, the robustness of mutations plays a role. For example, codons that are further away from a stop codon are preferred so that a single point mutation cannot immediately lead to the termination of the protein sequence. With respect to the host, for example, mutations are preferred that provide an advantage with respect to the immune system, such as turning it off or preventing recognition by antibodies or by other immune cells. This results in the viruses being able to replicate better and they cannot be wiped out as quickly by the immune system. But there are also other mutations that can give the virus an advantage, for example in the entry process into the cell via improved receptor binding or in the ability to infect different tissues (tissue tropism). Host-to-host transmission also plays a role. Viruses for which transmission works well naturally have an advantage.
ZOOP: You have just mentioned the interaction between the virus and the immune system of the host. In the context of the SARS-CoV-2 pandemic, the word "escape mutant" can often be read in the media. Can you explain what is meant by that?
Steffen: This describes a mutation that allows the virus not to be seen by the immune system. The immune system is made up of many different factors. There are antibodies, immune cells, and various other molecules of innate immunity in cells that all first serve to sense a viral infection and tell the body through signaling pathways that there is a viral infection. The immune system is alerted to take action against the infection. Antibodies recognize specific regions on proteins called epitopes. When a mutation occurs in the region of the epitope that changes the structure of the protein so that antibodies of a certain type can no longer bind to it, we speak of an "escape mutation." Of course, it is still possible for it to be recognized by antibodies that bind to other regions of the protein.
"It's basically a tug-of-war between the host and the virus"
ZOOP: You mentioned earlier that co-evolution of virus and host can occur. What does co-evolution mean in this case?
Steffen: Co-evolution occurs when viruses cause not only short, acute infections but also longer-term infections and adapt to the host. For example, under pressure from the host's immune system, viruses can change in such a way that they are no longer perceived as strongly by the immune system. This is seen with hepatitis viruses, among others. Here, the acute infection may already have occurred many years before the disease is recognized. This is due to the fact that the viruses can exist in the body undetected for a long time. A phenomenon we also see with viruses that cause less severe diseases. Herpes viruses are an example. In these viruses, we can observe certain adaptations to the host. Partly they develop mechanisms to interact with the host's immune system, downregulate or mimic certain parts. It's basically a tug-of-war between the host and the virus. The host wants to eliminate the virus quickly, while it's more in the virus' interest to be in the host for the long term rather than cause a severe or fatal infection.
"Many very dangerous viruses fall into the class of zoonotic viruses"
ZOOP: So the virus is not aiming to kill us. That's reassuring. How is it that there are nevertheless deadly viruses?
Steffen: It's mainly because it's not the right host. We observe this especially with zoonotic viruses, which are transmitted from animals to humans. Here, humans are not the appropriate host for the virus and it is more of a side effect that humans get sick or possibly die. It is actually not in the interest of the virus. With these viruses there are so-called reservoir hosts in which the virus population is present in the long term. The reservoir hosts maintain the virus population and usually show no symptoms of disease, although they may carry large numbers of copies of the virus. This is because co-evolution has progressed so far that virus and host are relatively well adapted to each other. The virus can no longer cause much damage and the host tolerates the virus to a large extent, so that the virus can replicate. In the case of a less suitable host, it is usually the immune system that becomes alarmed, leading to disease and severe courses. Often the viruses cannot control the immune system of the new host well, thus sometimes there is too strong an immune response, which can become dangerous.
ZOOP: What can make zoonotic viruses particularly dangerous to humans.
Steffen: Yes, many very dangerous viruses fall into the class of zoonotic viruses.
ZOOP: You have already mentioned that the genome of viruses is reduced to the essential. Nevertheless, we also talk about variable and conserved regions. Can you explain what is meant by that?
Steffen: It refers to the fact that not all mutations can prevail within a population. Mutations arise randomly at random positions in the genome. But there are certain regions where mutations are better tolerated, where it may even give the virus an advantage, through better fitness or receptor binding or the like. In terms of substitution rate, mutations are found more frequently in these variable regions. In other regions of the genome, mutations are not tolerated as well, such as regions that code for enzymes that have a specific function. If mutations occur here, the function of the enzyme can be impaired. Therefore, fewer mutations are found in these conserved genome segments, not because they do not arise there, but because they cannot establish themselves in the population.
"With SARS-CoV-2, large amounts of virus are already found in the upper respiratory tract, making transmission easier"
ZOOP: Now that we have already learned quite a bit about viral mutations, I would like to come back to SARS-CoV-2 at the end of the conversation. If you compare SARS-CoV-2 and SARS-CoV-1, what changes led to one virus being able to cause a pandemic and the other not?
Steffen: The most attention is on the surface protein, the spike protein. Here there is a cleavage site for proteases that is present in SARS-CoV-2, the so-called furin cleavage site. This is already known from other viruses. These surface proteins must be activated in order to mediate cell entry. Different viruses have developed different strategies. For coronaviruses, cleavage by proteases plays a role. The new cleavage site in SARS-CoV-2 leads to a higher activation of the surface proteins, in various cells, including lung cells.
In addition, transmission is different because SARS-CoV-2 is also found in the upper respiratory tract. In the case of SARS-CoV-1, the highest amount of virus was present in the lungs, which led to severe pneumonia and thus to the higher number of deaths per infections. However, this made the virus less transmissible because the virus first had to be coughed up deep from the lungs. With SARS-CoV-2, large amounts of virus are already found in the upper respiratory tract, making transmission easier. That alone was enough to trigger a pandemic.
"It was to be expected that there would be mutations"
ZOOP: SARS-CoV-2 is at least not more deadly, but more easily transmitted, than SARS-CoV-1. Meanwhile, there are already some SARS-CoV-2 mutants of concern. These include the B1.1.7 mutation first found in the UK, the P1 variant found in Brazil, and a mutant discovered in South Africa. Are you surprised that so many mutants have appeared, or was that, in your view, expected for an RNA virus?
Steffen: I think it was to be expected. The population size that plays a role is of course huge, given a worldwide circulation of the virus. So, it was to be expected that there would be beneficial mutations that manifest within the population. But of course, it's also worrisome. Even though we expect that the virus will adapt a little bit to the host, it's also possible that escape mutations will be able to become entrenched in the population that will allow the virus to escape the human immune system. So, expected yes but also worrisome. I think it's right and important that this is being monitored and evaluated more closely now.
"we already have a relatively good infrastructure in Germany, but it now needs to be expanded and promoted in a targeted manner "
ZOOP: Do you think Germany should do more for molecular surveillance?
Steffen: I think more should be done. In international comparison, Germany has never been in a particularly good position. But of course, the whole thing has financial limits and you have to see what can be invested here. I do believe that this is an area in which we should and must invest. I think it is urgently needed. In the future, I imagine that the determination of complete genome sequences will become standard diagnostics and that patients will then be able to be treated more individually. There is certainly still a long way to go, but I think the pandemic is a good impetus for Germany to expand its capacities in this area.
ZOOP: Perhaps we can also hope for technical advances in this area that will make sequencing easier and cheaper in the future.
Steffen: Yes, I assume so. There is a lot of progress in this area. But we are still a step away from that. We have to work with what we have for the time being. I think we already have a relatively good infrastructure in Germany, but it now needs to be expanded and promoted in a targeted manner.
"Mutations can always go both ways and don't always necessarily have to have negative effects"
ZOOP: As a final question, let me ask you about mutations in general. In public, the term "mutation" often has negative connotations. What do you think is the reason for that? After all, it is a very common thing.
Steffen: I think it's because we talk about mutations mostly in the context of diseases. Now with viruses we talk about mutations making the virus more dangerous and also in relation to cancer we talk about mutations resulting in heritable gene changes and defects. This probably creates the undertone that mutations are something negative. But of course, one should also keep in mind here that mutations can also cause a virus to become less dangerous, to adapt better to the host, or to be more easily warded off. Mutations can always go both ways and don't always necessarily have negative effects.
ZOOP: A nice conclusion to the interview: "Mutations are not always negative." Dr. Steffen, thank you very much for the interview!
Interview: Dr. Dana Thal, German Research Platform for Zoonoses